Evidence Based Medicine is the concept of treating patients based on solid evidence. This is in contrast to the previous practice of treatment based on individual experiences and extrapolation from known scientific data.
Considering the long history of medicine, the concept of evidence based medicine is relatively new. Although the first clinical trial is attributed to James Lind in 1747, it wasn't until the mid-twentieth century that the concept of Randomized Controlled Trials (RCT) for clinical evidence took root. The term Evidence Based Medicine wasn't coined until 1991ebm-hist. However the concept is now almost universally accepted in the academia.
Evidence
An evidence is something we know confidently and to the best of our abilities. Appropriate evidence requires careful elimination of biases and it must go through a rigorous process of quality checking to be accepted.
Evidence doesn't mean infallible truth though, and as our knowledge evolves evidences may sometime change. So the evidences are better described as the best available information at a given point in time, justified for clinical use.
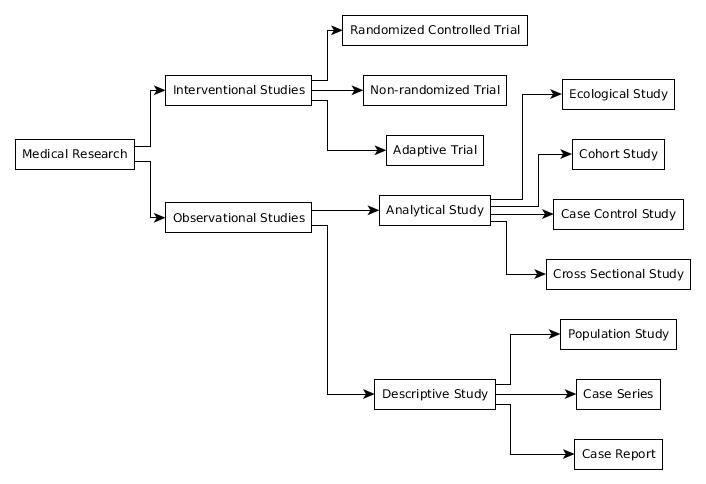
Hierarchy of Evidence
There are many types of studies and not all of them were created equal. While studies like meta-analysis are very high quality evidences, case reports are rather weak in this matter. Based on their quality, a list can be made with the best at the topgreenhalgh —
- Systematic Reviews and Meta-analysis
- Randomized Controlled Trials
- Cohort Studies
- Case Control Studies
- Cross-sectional Studies
- Case Series
- Case Reports
Quality Assessment of Studies
In order to determine the importance of a study in a particular condition it is essential to assess their quality. Quality assessment is usually performed with the help of some predetermined tools, usually in the form of a questionnairezeng.
- Systemic review and meta analysis
- AMSTAR tool, CASP checklist
- Randomized Controlled Trials
- Risk of Bias tool by Cochrane Collaboration, modified Jadad scale, Delphi list, PEDro scale, CASP checklist, NICE methodology checklist etc.
- Non-randomized trials
- ROBINS-I tool, MINORS tool, Reisch's tool etc.
- Cohort and case-control studies
- CASP checklist, SIGN methodology tools, Newcastle-Ottawa scales etc.
- Cross-sectional studies
- AXIS tool
- Case series
- Modified Delphi technique
- Diagnostic Test Accuracy studies
- QUADAS tool, CASP checklist etc.
- Clinical practice guidelines
- AGREE instrument
The GRADE System
Obviously calculating the real importance of different studies is much more complicated than just arranging them in a hierarchy. This especially is a big problem while designing a guideline. So to make the process uniform a new system was developed in early 2000, The Grading of Recommendations Assessment, Development and Evaluation or The GRADEgrade.
In this system a grade is assigned to a particular study like thisgrade-paper —
- Initial grade assignment:
- Randomized controlled trial — high
- Observational study — low
- Any other study — very low
- Grade further decreased if —
- Serious limitation to study quality
- Important inconsistency
- Uncertainty about directness of study question
- Imprecise or sparse data
- High risk of bias
- Grade further increased if —
- Strong evidence of association
- Evidence of dose-response gradient
- All plausible confounders, if present, would have reduced the effect
Levels of Evidence
After assessing all available evidences for a particular condition, the evidences are usually presented in terms of levels. One of the most common system is this oneburns —
- Level 1
- Level 1A : Systematic Review of randomized controlled trials
- Level 1B : Individual randomized controlled trials
- Level 1C : All or none studies (usually anecdotal data)
- Level 2
- Level 2A : Systematic Review of cohort studies
- Level 2B : Individual cohort study / low quality randomized controlled trials
- Level 2C : Ecological study / outcomes research (usually outcome of public health interventions
- Level 3
- Level 3A : Systematic review of case control studies
- Level 3B : Individual case control study
- Level 4 : Case series / poor quality cohort or case-control study
- Level 5 : Expert opinions
Practice Recommendations
Based on these evidences different grades of recommendations can be made for practical use in the clinics, ranging from stronger to weaker recommendationsburns.
- Grade A : Strong recommendation
- Level 1 or highly consistent findings from multiple level 2, 3 or 4 studies
- Grade B : Recommendation
- Level 2, 3 or 4 with generally consistent findings
- Grade C : Optional
- Level 2, 3 or 4 with inconsistent findings
- Grade D : Optional
- Level 5 evidence
Guidelines
It is practically impossible for every clinician to examine every available evidence in the literature to apply them to his/her clinical practice. And even if they tried to do so it would result in great inconsistency between the practices of different clinicians.
Guidelines are made to avoid this problem. Generally public health departments or medical organizations make these guidelines based on available evidences. They are developed in several steps which includelim —
- Defining the clinical question
- Systematic search of the literature
- Critical appraisal of the studies and grading of evidences
- Summarization of the evidences into the guideline
However guidelines are not unbreakable rules. Individual clinicians often need to adapt it depending on the case at hand. Besides the guidelines change with time as more and more evidences accumulate. Sometimes even different guidelines are issued by different organizations more often than not.
Publications
Our source of evidence is mostly the medical journals that publish different study results. The reputable journals are usually very picky about what they publish, and if a paper makes it through the publication hurdles it is considered worthy of attention.
Peer Review
The standard of quality control in the academia at present is a process known as peer review. It means that the quality of a paper can only be judged by fellow clinicians who are expected to judge the merit of the paper impartially.
After submission of a paper to a journal it is usually sent to two or more peer reviewers outside the journal. These peer reviewers then read the paper and then accept or reject it or suggest modifications.
Peer reviewers are almost always unpaid volunteers to avoid any conflict of interest. Ideally the peer review process should be double blinded so that the identity of the author doesn't influence the reviewers' decision.
Indexing
There are thousands of medical journals all over the world and it is impossible to keep track of all the articles published in each of them by individuals. This is why we need indexing services which tracks the journals for us and presents the articles at a single access point. The most popular of these indexing services is the PubMed which indexes about 30,000 journals worldwide. Being indexed in PubMed is often seen as a sign of prestige for a journal. Other indexing services include EMBASE, Google Scholars etc.
The indexing is important in our discussion because most systematic reviews and meta-analysis search literature from these indexing services. So if a journal is not indexed then the articles published there may be missed from the evidence base.
Publication Bias
All reputable journals are usually very picky about what they publish. If a published article gets cited by other articles, then the standing of the journal gets a little improved (e.g. the impact factor). This is why journals prefer articles that are likely to be cited, such as studies with positive findings. This means some studies with negative results are destined to remain in a dusty drawer for eternity, never to be published. Some papers like the conference presentations, student dissertations etc. often do not get published at all.
This phenomenon is called as publication bias and it causes a serious loss of valuable evidences. As a result the evidence may get skewed towards the positive results. This must be taken into account when conducting a systemic review and meta-analysis and one of the steps involves plotting a funnel plot to determine the degree of publication bias.
Justification
Despite several drawbacks of the evidence based medicine, which we will discuss shortly, it has some excellent advantages over the old approach. The old approach was based on individual experiences and logical reasoning based on what is known. These methods have several flaws. First, experiences of individual clinicians vary greatly between different persons. They are also very much prone to bias. Logical reasoning also have problems. For example the reasoning for a medical intervention may be based on what we know about the physiology of the body and the chemical properties of the drug. Even if we test it on a few patients and find it to be apparently working, it doesn't say about the efficacy much. Every individual is different and we can't know if a drug works until we perform sufficiently large clinical trial with the drug.
This is the importance of evidence based medicine. It's pretty simple, we need know whether something actually works, before saying that it works. Most importantly we give higher priority to the good quality study results over anecdotal references and opinions of individual clinicians.
It gives us several advantages in my opinion —
- It takes into account the variability in the population
- Tries to eliminate bias, such as blinding in randomized controlled trials
- Uniformity in clinical practice
- Leaves nothing to the imagination
Pitfalls
As everything in this world, evidence based medicine is not flawless either. Several drawbacks can be identified which have often been used to criticize this concept.
- Evidence based medicine is slow. It takes time to accumulate evidences and its often impossible to keep up during the hours of crisis, such as a pandemic.
- Health infrastructure varies greatly between regions, especially in remote areas. It may often be impossible to follow guidelines in such areas, due to lack of materials or for other reasons.
- Loss of evidence in the form of unpublished studies also severely threaten the foundation of the evidence based medicine.
For Noobs
This section is only for those who are unfamiliar with the literature search. This is more of a cheat-sheet than a comprehensive guide because nobody likes to read large blocks of text that uses many words to say little.
I'll divide the whole quick-start process of how to read a paper in a number of phases.
- Phase 1 : Get familiar with PubMed
- PubMed is the best literature index out there and you'll need it for almost everything. So go over there and start exploring. It has a search bar just like google and when you search something it gives you a list of results. If you look closely you'll find a sort by option. Two of them are most important, sort by most recent will give you a list of articles arranged according to the date of entry, you'll need this option if you have thorough and exhaustive literature search in mind. On the other hand sort by best match is a better option if you want to find something specific in a short time.
- Phase 2 : Abstracts are your best friends
- Abstracts are just summaries of the whole papers. PubMed will show you abstracts in most cases. You'll find most important things about the paper in these abstracts and more often than not your question will be answered by the abstracts alone.
- Phase 3 : Access the full text
- Accessing the full text is easy if the journal is open access, PubMed will show you the download link. However, if the journal is closed source you have limited options, you can either pay for it or if your institution has institutional access, then you can use the library. Sounds bad? Well, there is another way, although of questionable legality. Sci-Hub will get almost any paper for you, for free.
- Phase 4 : Start at the bottom
- Start with the conclusion first, you'll get a nice idea of what the authors did and why they did so. Now move on to the discussion. They are pretty large in most papers, but if you manage to read it you'll find out almost everything about the paper. You see, these two sections are the most important part of the study (well, arguably).
- Phase 5 : The technical part
The results and the methods sections are the intricate machineries of a study. Once you get a hang of reading the discussion section you will be able to target these parts. You'll need a fairly good understanding of medical research methodology to really understand them and it takes a fairly long time. So don't feel bad if it looks like Chinese to you (or some other language if you speak Chinese). Go find that PSM book your professors tormented you with, ask people who know these stuffs and promise them treat but most importantly use google and be patient.
Another trick is to just keep reading them even if you don't understand it right away, eventually you'll discover a pattern that keeps repeating. Once you find these patterns you'll be able to easily decipher them with a little help from Google.
- Phase 6 : No longer a noob
- Congratulations! You are no longer a noob. PubMed and other indexing services are now at your disposal. Go explore whatever you want, maybe write some papers of your own. But don't forget to learn how to critically appraise a paper. You can't have evidence based medicine without quality assessment.
Links
- Evidence Based Medicine at BMJ
- Center for Evidence Based Medicine
- Cochrane Risk Bias Tools
- CASP Checklists
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. The history of evidence-based medicine. 2016 Jun 15 [Updated 2016 Sep 8]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK390299/
- Greenhalgh T. How to read a paper. Getting your bearings (deciding what the paper is about). BMJ. 1997;315(7102):243‐246. https://doi.org/10.1136/bmj.315.7102.243
- The GRADE Working Group. GRADE Homepage. Avialable from: https://www.gradeworkinggroup.org/
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004 Jun 19;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490. PMID: 15205295; PMCID: PMC428525.
- Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011 Jul;128(1):305-10. https://doi.org/10.1097/PRS.0b013e318219c171. PMID: 21701348; PMCID: PMC3124652.
- Lim W, Arnold DM, Bachanova V, Haspel RL, Rosovsky RP, Shustov AR, Crowther MA. Evidence-based guidelines--an introduction. Hematology Am Soc Hematol Educ Program. 2008:26-30. https://doi.org/10.1182/asheducation-2008.1.26.
- Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015 Feb;8(1):2-10. https://doi.org/10.1111/jebm.12141.
 RSS Feed
RSS Feed
Write a comment: