In a healthy individual the pH of the arterial blood is maintained within 7.35 to 7.45. The two major systems responsible for maintaining acid base balance are the respiratory system and the renal system. Loss of this balance has deleterious effects on health and often indicative of some underlying disorders.
The Basics
Henderson-Hasselbalch Equation
The pH of the arterial blood depends on the concentration of bicarbonate (HCO₃) and the partial pressure of CO₂ (PaCO₂) in the blood. The pH is calculated by the Henderson-Hasselbalch equation.
`pH = 6.1 + log([HCO₃⁻] / (PaCO₂ × 0.0301))`
Maintenance of Acid Base Balance
During normal metabolic process H⁺ and CO₂ are generated in the tissue. There are 3 mechanisms that prevents them from altering the pH of the blood.
Buffers
Buffers are a mixture of weak acid and its salt that resists change of pH if strong acid or alkali is added to the media.
- Intracellular Buffers
- Proteins
- Phosphates
- Extracellular Buffers
- Bicarbonates
- Hemoglobin
Respiratory System
The CO₂ produced in the tissue is dissolved in the plasma and transported to the lung as H⁺ and HCO₃⁻ ions. In the lungs they combine again to form H₂O and CO₂.
`H⁺ + HCO₃⁻ ⇋ H₂CO₃ ⇋ H₂O + CO₂`
This CO₂ is ventilated out by the respiratory system.
If there is accumulation of CO₂ due to hypoventilation the equilibrium is tilted towards left and the extra H⁺ ion decreases the pH causing acidosis. On the other hand if there is increased CO₂ washout due to hyperventilation the equilibrium is tilted towards right and there is an alkalosis.
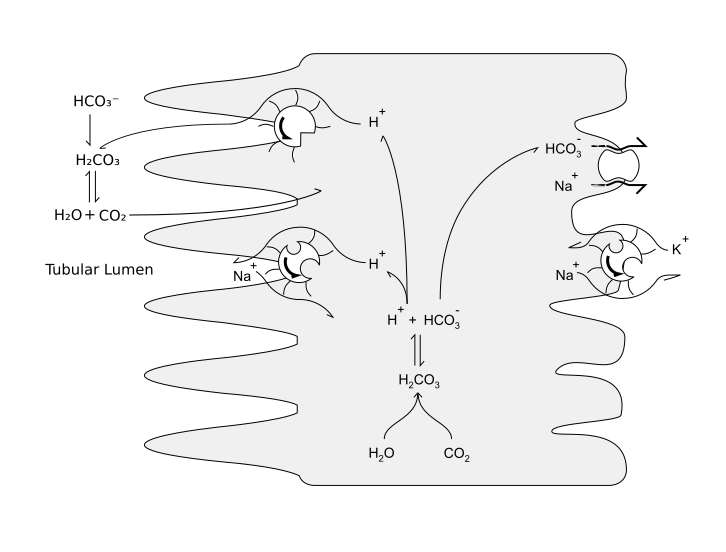
Renal System
HCO₃⁻ is freely filtered in the glomerulus, but H⁺ ions can not pass through glomerulus because they are bound with proteins.
Normally all of the filtered HCO₃⁻ is reabsorbed in the tubular system, majority (80%) of which are reabsorbed in the proximal tubule.
H⁺ is actively secreted in the tubules with the help of Na⁺/H⁺ ATPase pump and H⁺/K⁺ ATPase pump. In the PCT one HCO₃⁻ is reabsorbed for every H⁺ secreted and in the CD one new HCO₃⁻ is generated for every H⁺ secreted.
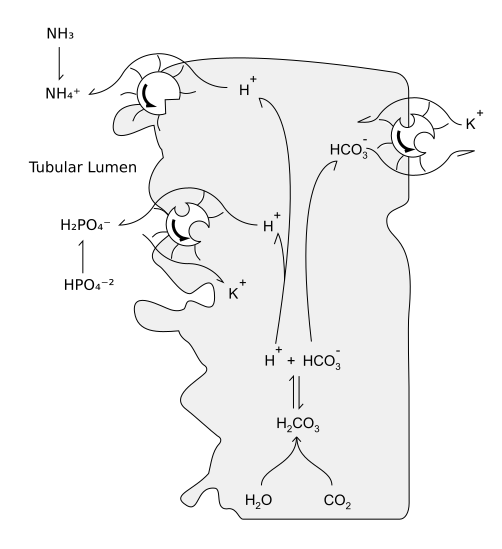
Majority of the H⁺ in the urine is buffered by NH₃ in the form of NH₄⁺ and the rest is secreted as titratable acid.
Normal pH of urine is around 5.5 due to secretion of acid. The limiting pH of urine is 4.5 at which point no more acid can be secreted in the urine.
Courtesy: ColnKurtz (CC BY-SA 4.0)
Courtesy: ColnKurtz (CC BY-SA 4.0)
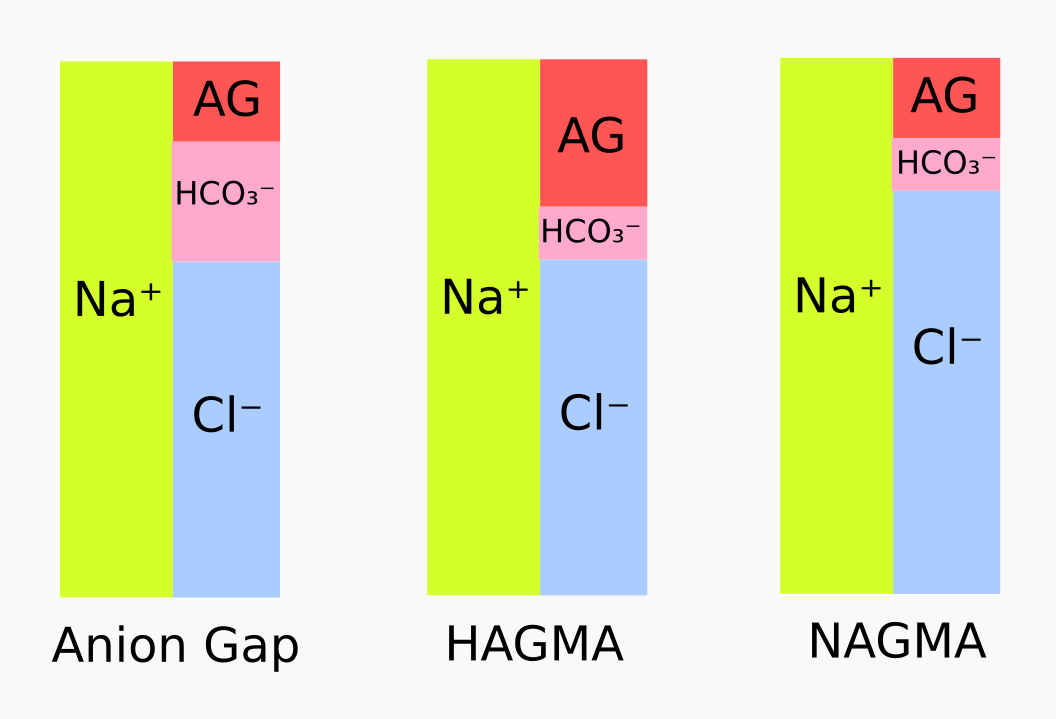
Anion Gap
Blood contains an equal amount of anions and cations, but not all of them are measured. The difference between the measured anions and the measured cations is known as anion gap. It is calculated as follows —
`AG = [Na⁺] - ([Cl⁻] + [HCO₃⁻])`
The main component of unmeasured anion is albumin, so in the presence of gross changes in the plasma albumin concentration , anion gap needs to be adjusted —
`AG_"correct" = AG + {(4 - ["albumin"]) × 2.5}`
Anion gap helps in determining the cause of metabolic acidosis. An increased anion gap indicates accumulation of an unmeasured acidic anion. On the other hand a normal anion gap indicates loss of HCO₃⁻ which is usually associated with reciprocal increase in Cl⁻ concentration.
Base Excess
Base excess is defined as the amount of strong acid necessary to return the pH of the blood to 7.4 under standard conditions (fully oxygenated, partial pressure of CO₂ at 40 mmHg and temperature at 37℃).
Normal range of base excess lies within -2 to +2. Base excess is > +2 in metabolic alkalosis and < -2 in metabolic acidosis.
Since the change in PaCO₂ is accounted for, the base excess measures solely the metabolic contribution in a acid base disorder.
Base excess can be calculated from the HCO₃⁻ level in the blood —
`BE = (0.93 × [HCO₃⁻]) + (13.77 × pH) - 124.58`
Standard Base Excess is the base excess measured after standardizing the Hb level at 5 gm/dl. It closely mirrors the acid base status of the ECF and hence more useful in assessing the condition.
Normal Values
| Parameter | Normal value |
|---|---|
| pH | 7.35 - 7.45 (mean 7.4) |
| PaO₂ | 80 - 100 mmHg |
| SaO₂ | ≥ 95% |
| PaCO₂ | 35 - 45 mmHg (mean 40 mmHg) |
| HCO₃⁻ | 18 - 30 mmHg (mean 24 mmHg) |
| Anion Gap | 10 - 12 mmol/L |
| Base Excess | -2 to +2 meq/L |
Acid Base Disorders
There are 4 basic types of Acid Base disorders that may result from a disruption of the normal acid base balance.
Types of Acid Base Disorders
-
Metabolic Acidosis: It is characterized by ↓HCO₃⁻. It is usually caused by —
- ↑ Acid production
- ↑ Acid accumulation
- ↑ HCO₃⁻ loss
It has two types —
- High Anion Gap Metabolic Acidosis (HAGMA)
- It is caused by a reduction in the plasma HCO₃⁻ but no change in the plasma Cl⁻.
- 4 main reasons are — lactic acidosis, ketoacidosis, toxins and acute / chronic renal failure.
- Normal Anion Gap Metabolic Acidosis (NAGMA)
- The reduction in the plasma HCO₃⁻ is matched by increase in the plasma Cl⁻ which keeps the AG normal.
- 2 main reasons are — GI loss of HCO₃⁻ and renal loss of HCO₃⁻.
-
Metabolic Alkalosis: It is characterized by ↑HCO₃⁻. It may be caused by —
- Loss of H⁺
- Plasma volume depletion (contraction alkalosis)
- HCO₃⁻ administration
Respiratory Acidosis: It is characterized by ↑PaCO₂. It is usually caused by hypoventilation and CO₂ retention.
Respiratory Alkalosis: It is characterized by ↓PaCO₂. It is usually caused by hyperventilation and CO₂ washout.
Compensation
Normally there is an equilibrium between HCO₃⁻ and PaCO₂. Change in one causes a reciprocal change in the other. This change is called compensation. Compensation limits the change in pH by altering it in the opposite direction towards normal. However pH can never return to normal due to compensation.
In case of metabolic disorders respiratory compensation is always present. However metabolic compensation is only present in chronic respiratory disorders, and not in acute respiratory disorders.
| Disorder | pH | Primary change | Compensatory change |
|---|---|---|---|
| Metabolic acidosis | ↓ | ↓HCO₃⁻ | ↓PaCO₂ |
| Metabolic alkalosis | ↑ | ↑HCO₃⁻ | ↑PaCO₂ |
| Respiratory acidosis | ↓ | ↑PaCO₂ | ↑HCO₃⁻ |
| Respiratory alkalosis | ↑ | ↓PaCO₂ | ↓HCO₃⁻ |
Mixed Disorders
Multiple acid base disorders can coexist in a patient. It can be identified by unexpected change in parameters not explained by compensation. Sometimes mixed disorders may cause pH to remain normal, such as metabolic acidosis + respiratory alkalosis, metabolic acidosis + metabolic alkalosis etc. Careful examination of other parameters like PaCO₂, HCO₃⁻, AG etc. provides clues for identification of such conditions.
Other Electrolytes
-
Potassium (K⁺)
Usually there is an increase in the plasma K⁺ concentration (hyperkalemia) in acidosis, especially metabolic acidosis except those due to organic acids.
In NAGMA a large amount of H⁺ ions migrate from plasma into the cells where they are buffered by intracellular buffers. It occurs with the help of H⁺-K⁺ ATPase pump which exchanges K⁺ ions for H⁺ ions. This phenomenon causes shift of K⁺ from cells to plasma and raises plasma K⁺ concentration. It is known as internal potassium balance.
In HAGMA extracellular shift of K⁺ by this mechanism is less pronounced. The increase in plasma K⁺ is often due to other mechanisms, e.g. chronic renal failure, insulin deficiency in diabetic ketoacidosis etc.
There is also an external potassium balance which is regulated by the kidneys. The increased plasma K⁺ decreases K⁺ reabsorption in renal tubules and increases renal K⁺ loss, which may result in hypokalemia after correction of acidosis.
-
Chloride (Cl⁻)
In NAGMA decrease in plasma HCO₃⁻ is matched by an increase in the plasma Cl⁻. For example in diarrhoea Na⁺ loss is more than Cl⁻ loss through GI fluid. HCO₃⁻ is also lost in diarrhoea. In this scenario, particularly if NaCl infusion is given, the increased Cl⁻ effectively replaces the HCO₃⁻ causing acidosis.
Hypochloremia is often responsible for the maintenance phase of metabolic alkalosis. In absence of Cl⁻ ions, there is increased reabsorption of HCO₃⁻ to maintain the electro-neutrality resulting in alkalosis.
-
Calcium (Ca⁺²)
In alkalosis there is an increase in the protein bound fraction and consequent reduction in free Ca⁺² ions.
Arterial Blood Gas Analysis
ABG is the most important test to assess the acid base status of a patient.
Indications
- Conditions that can lead to metabolic acidosis.
- E.g. Diabetic ketoacidosis, renal failure, cardiac failure, hepatic failure, sepsis, burns etc.
- Acute / chronic respiratory failure
- Post-operative patients after coronary artery bypass grafting
- Stable patients in ICU
- Patients receiving supplemental low flow oxygen
Procedure
-
Heparinize the syringe: The syringe to be used for blood sample collection is heparinized with 0.05 - 0.01 ml of heparin per ml of blood. Usual required amount of blood is 3 ml, so 0.15 - 0.3 ml of heparin is needed to be drawn into the syringe.
Too much heparin causes the pH to be spuriously low. On the other hand too little heparin may result in clotting of the sample.
-
Choose the site: The preferred site of collecting arterial blood sample is the radial artery in the wrist.
A common adverse effect of this procedure is vasospasm which may compromise the blood flow through the radial artery. However the vascularity of the palmar arch is adequately maintained by the ulnar artery. Therefore it's necessary to confirm the adequacy of ulnar artery by performing the modified Allen's test before collecting blood sample.
Modified Allen's Test: The test is performed as below —
- Ask the patient to make a fist.
- Occlude both radial and ulnar artery with your fingers.
- Ask the patient to open the fist. The palm should be blanched.
- Release the ulnar artery.
- Look for the flushing of the palm. Flushing within 10 - 15 seconds indicates a positive modified Allen's test.
A positive modified Allen's test indicates that blood flow through ulnar artery is adequate and it's safe to collect blood from the radial artery.
-
Collect the sample: The steps for collecting arterial blood sample are as follows —
- Extend the patient's wrist.
- Feel the pulsation of the radial artery and mark the site.
- Puncture the site with the heparinized syringe at an 45° angle.
- Appearance of a bright red column of pulsating blood at the indicator of the syringe confirms that the needle has entered the artery.
- Draw 3 ml of blood.
- Withdraw the needle and maintain pressure over the puncture site.
- Analyze the sample: The sample is placed in an ABG auto-analyzer. The output is recorded after the analysis is complete. A simultaneous sample may also be drawn for the electrolyte panel.
-
Check for validity: The SaO₂ value obtained from the ABG analysis should correspond within 2% of the SpO₂ value measured with the pulse-oximeter.
SaO₂ << SpO₂ indicates venous or mixed blood sample.
SaO₂ >> SpO₂ indicates presence of abnormal hemoglobin e.g. CO poisoning, cyanide poisoning, methehemoglobinemia etc.
Parameters
The following parameters are necessary for the interpretation of ABG analysis —
- pH
- PaCO₂
- HCO₃⁻
- AG
Other Tests
The following values obtained from other tests also provide helpful clues in diagnosis —
- Osmolar Gap
- Urinary Anion Gap
- Urinary Chloride
Interpretation of ABG Analysis
ABG analysis can be interpreted in following 4 steps —
Exclude Hypoxemia
A PaO₂ value of ≤ 60 mmHg indicates hypoxemia in the patient. The alveolar-arterial gradient (A-a gradient) helps in diagnosis.
`"A-a gradient" = (150 - 5/4 (PaCO₂)) - PaO₂`
The normal value of A-a gradient is 5-25 mmHg.
- Normal A-a gradient indicates an extra-pulmonary cause. Further clue can be obtained by measuring PaCO₂.
- ↓ PaCO₂ ⇒ Low FiO₂ e.g. hight altitude
- ↑ PaCO₂ ⇒ Hypoventilation disorders
- ↑ A-a gradient indicates a pulmonary cause. The patient's response to O₂ therapy provides further clue.
- Normalizes on O₂ therapy ⇒ V:Q mismatch disorders e.g. chronic obstructive lung disease, interstitial lung disease, pulmonary thromboembolism etc.
- Remains high on O₂ therapy ⇒ Alveolar defect e.g. pneumonia, pulmonary edema, ARDS etc.
- Intra-cardiac shunting is the only extra-pulmonary cause of hypoxemia with an ↑ A-a gradient.
The gold standard test for hypoxemia is ABG analysis whereas pulse oximetry is the best screening test.
Hypoxemia is treated with O₂ supplementation and management of the underlying cause. The single best prognostic factor for hypoxemia is the severity of pulmonary artery hypertension.
Identify Presence of Acid-Base Disorders
Abnormal pH value indicates the presence of an acid-base disorder.
- pH < 7.35 ⇒ acidosis
- pH > 7.45 ⇒ alkalosis
However a normal pH doesn't guarantee the absence of an acid-base disorder. It is also necessary to check the values of HCO₃⁻, PaCO₂ and AG to see if they are within normal limits.
Abnormal values of PaCO₂ and HCO₃⁻ in the presence of normal pH indicates a mixed acid base disorder.
Find Primary Disorder
The change in pH is accompanied by a change in HCO₃⁻ and PaCO₂. In case of simple disorders, the change explaining the pH alteration is the primary disorder and the other is the compensatory change.
| ↓ pH | ↓ HCO₃⁻ ⇒ Metabolic Acidosis |
| ↑ PaCO₂ ⇒ Respiratory Acidosis | |
| ↑ pH | ↑ HCO₃⁻ ⇒ Metabolic Alkalosis |
| ↓ PaCO₂ ⇒ Respiratory Alkalosis |
Simple vs Mixed
It is possible to have two or more coexisting disorders. They can be identified by comparing values of the parameters with their expected changes.
Respiratory acidosis and respiratory alkalosis usually does not coexist.
Metabolic Disorder + Respiratory Disorder
Disorder in one system is expected to be accompanied by compensatory change in the other. Deviation of HCO₃⁻ or PaCO₂ from the expected values suggests a mixed disorder.
| ↓ HCO₃⁻ | PaCO₂>expected | Metabolic Acidosis + Respiratory Acidosis |
| ↓ HCO₃⁻ | PaCO₂<expected | Metabolic Acidosis + Respiratory Alkalosis |
| ↑ HCO₃⁻ | PaCO₂>expected | Metabolic Alkalosis + Respiratory Acidosis |
| ↑ HCO₃⁻ | PaCO₂<expected | Metabolic Alkalosis + Respiratory Alkalosis |
The expected values can be calculated by any of the 3 methods —
Winter's Formula
`PaCO₂ = (1.5 × [HCO₃⁻]) + 8 ± 2`
Compensation Table
| Metabolic Disorder | Expected Δ PaCO₂ / Δ HCO₃⁻ | |
|---|---|---|
| Metabolic Acidosis | PaCO₂ ↓ 1.25 mmHg per mmol/L ↓ HCO₃⁻ | |
| Metabolic Alkalosis | PaCO₂ ↑ 0.75 mmHg per mmol/L ↑ HCO₃⁻ | |
| Respiratory Disorder | Expected Δ HCO₃⁻ / Δ PaCO₂ | |
| Acute | Chronic | |
| Respiratory Acidosis | HCO₃⁻ ↑ 0.1 mmol/L per mmHg ↑ PaCO₂ | HCO₃⁻ ↑ 0.4 mmol/L per mmHg ↑ PaCO₂ |
| Respiratory Alkalosis | HCO₃⁻ ↓ 0.2 mmol/L per mmHg ↓ PaCO₂ | HCO₃⁻ ↓ 0.4 mmol/L per mmHg ↓ PaCO₂ |
Acid-Base Nomogram
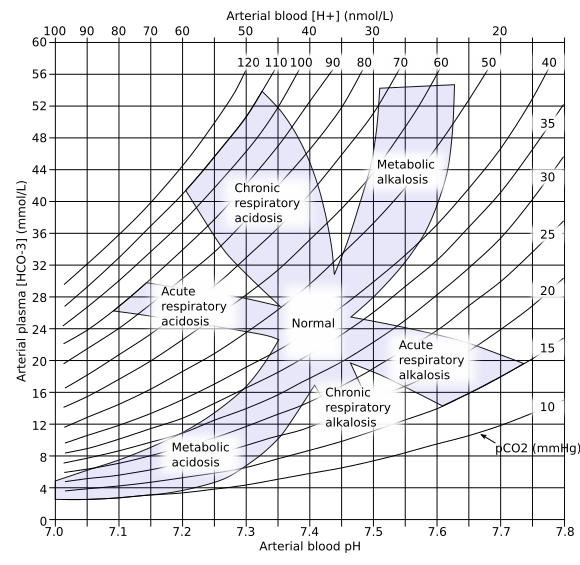
Courtesy: Huckfinne (Public Domain)
Mixed Metabolic Disorders
Mixed metabolic disorders can result from a combination of hight anion gap metabolic acidosis (HAGMA) and normal anion gap metabolic acidosis (NAGMA). Comparing change in anion gap with the change in HCO₃⁻ provides important clues in identifying mixed metabolic disorders.
| Δ AG = Δ HCO₃⁻ | Simple HAGMA | Change in AG is explained by change in HCO₃⁻ |
|---|---|---|
| Δ AG < Δ HCO₃⁻ | HAGMA + NAGMA | HAGMA and NAGMA both decreases HCO₃⁻, but only HAGMA changes AG |
| Δ AG > Δ HCO₃⁻ | HAGMA + Metabolic Alkalosis | HCO₃⁻ does not decrease as expected due to concurrent metabolic alkalosis. |
This method can not identify NAGMA + Metabolic Alkalosis as neither of them causes any change in AG.
Algorithm
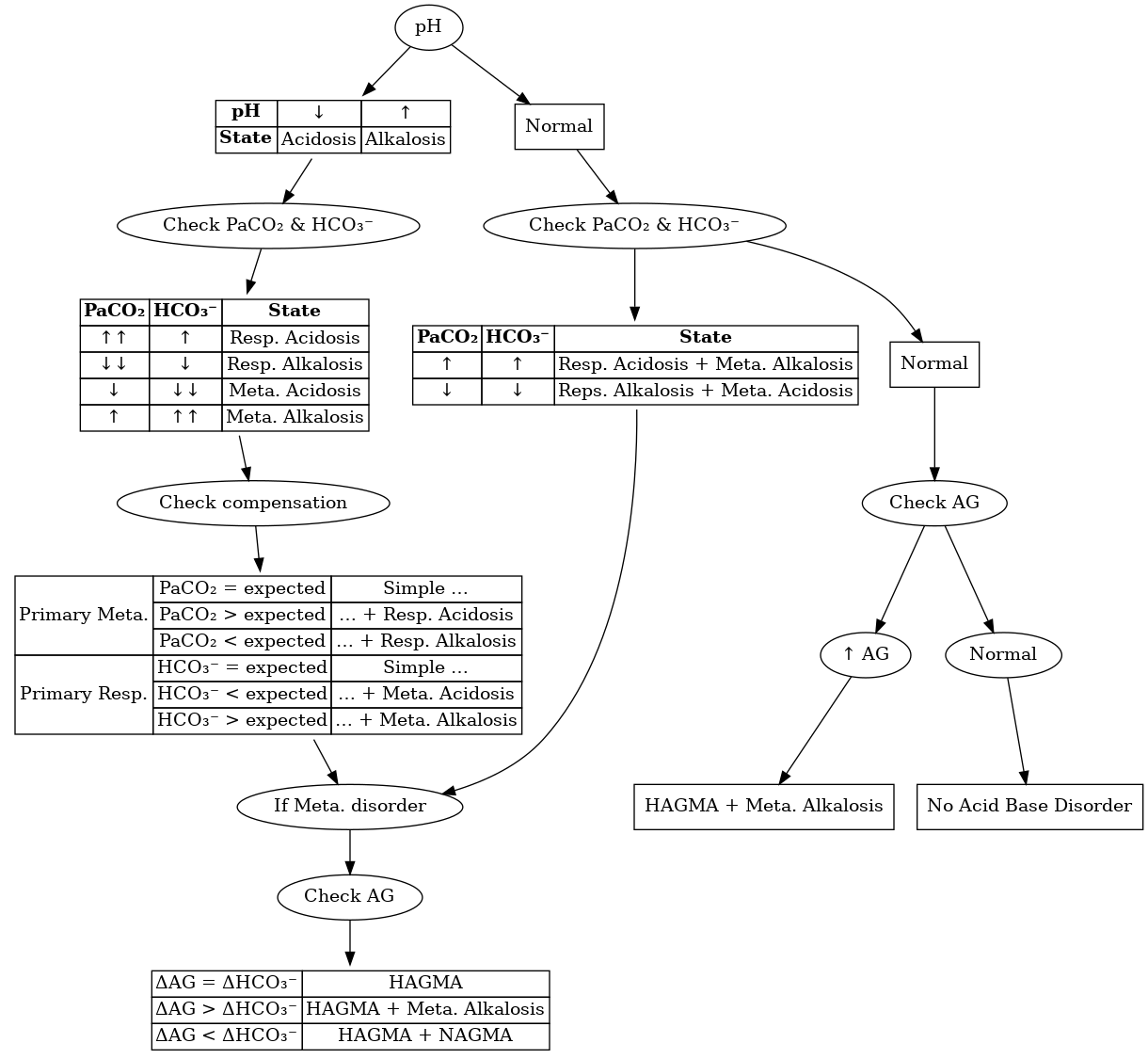
Individual Disorders
HAGMA
Characterized by ↓HCO₃⁻ and ↑AG.
Etiology
HAGMA occurs when there is an accumulation of acidic compound which also contributes to the unmeasured anions and thereby increasing the AG. It has 4 major causes —
- Lactic Acidosis
- Poor tissue perfusion (Type A)
- Circulatory insufficiency e.g. shock, cardiac failure etc.
- Severe anemia
- Mitochondrial enzyme defects and inhibitors e.g. CO, cyanide etc.
- Aerobic disorders (Type B)
- Malignancies
- NRTI in HIV
- Diabetes mellitus
- Renal or hepatic failure
- Thiamine deficiency
- Severe infections e.g. cholera, malaria etc.
- Seizures
- Drugs / toxins e.g. biguanides, ethanol etc.
- Poor tissue perfusion (Type A)
- Ketoacidosis
- Diabetic ketoacidosis (usually simple)
- Cessation of insulin therapy
- Increased demand of insulin e.g. infection, MI etc.
- Alcoholic ketoacidosis (usually mixed)
- May be mixed with respiratory alkalosis due to liver disease
- May be mixed with metabolic alkalosis due to vomiting
- Diabetic ketoacidosis (usually simple)
- Drugs & Toxins
- Salicylates (mixed with respiratory alkalosis)
- Alcohols
- Ethylene glycol (oxalate crystals in urine)
- Methanol
- Advanced renal failure
- NAGMA of moderate renal failure is converted to HAGMA in advanced renal failure
- Uremia, ↓NH₄⁺ in urine, ↑Ca⁺² excretion in urine
- Alkali salt from bone used up for buffering ⇒ loss of bone mass
Approach
- History of any predisposing condition or exposure to toxin
- ABG analysis
-
Find the osmolal gap:
Osmolal gap is defined as the difference between measured osmolality and the calculated osmolality. The following formula gives the osmolal gap —
`"Osmolal gap" = "Measured osmolality" - (Na⁺ × 2 + "glucose" / 18 + "BUN" / 2.8)`
Normal value of osmolal gap is 10 - 15 mmol/kg of H₂O
An increased osmolal gap in metabolic acidosis indicates presence of unmeasured osmolytes e.g. ethanol, ethylene glycol etc.
- Exclude respiratory alkalosis (salicylates)
- Exclude diabetes and its complications
- Exclude ketoacidosis (ketone body in blood)
- Exclude alcohol ingestion (β-hydroxybutyrate, can't be detected by nitroprusside test for ketone bodies)
- Exclude renal failure (uremia, BUN, creatinine)
- Exclude ethylene glycol poisoning (oxalate in urine)
- Look for conditions causing lactic acidosis
Treatment
- Lactic acidosis
- Correct underlying disorder
- Restore tissue perfusion
- Avoid vasoconstrictors
- NaHCO₃ can only be used in severe acidosis (pH < 7.15)
- NaHCO₃ infusion is given to raise the pH to 7.2 over 30 - 40 minutes. It should not be used to raise pH to normal.
- Too much NaHCO₃ infusion may cause overshoot alkalosis because lactates are converted to HCO₃⁻ when the underlying cause of acidosis is removed.
- Diabetic ketoacidosis
- IV fluid
- 2-3 L 0.9% saline over first 1-3 h
- Subsequently 0.45% saline at 250-500 ml/h
- 5% glucose and 0.45% saline 150-250 ml/h when plasma glucose reaches 250 mg/dl
- Care should be taken not to cause volume over-expansion as it may result in NAGMA
- IV short-acting insulin
- 0.1 units/kg initial dose
- 0.1 units/kg/h continuous infusion
- Increase 2-3 fold if no response by 2-4 h
- If K⁺ < 3.3mmol/L, avoid insulin until K⁺ is corrected
- K⁺ supplement
- If K⁺ < 3.5 meq/L → 40-80 meq/h
- If K⁺ < 5.2 meq/L → 10 meq/h
- Monitor capillary glucose, BP, pulse, respiration, fluid input-output, electrolytes, anion gap
- IV fluid
- Alcoholic ketoacidosis
- IV fluid (5% dextrose in 0.9% NaCl)
- Correct hypophosphatemia, hypokalemia, hypomagnesemia
- Hypophosphatemia usually emerges after 12 - 24 hours and may result in rhabdomyolysis
- Treat associated disorders e.g. upper GI hemorrhage, pancreatitis, pneumonia etc.
- Salicylate toxicity
- Gastric lavage with isotonic saline (not NaHCO₃)
- Activated charcoal through NG tube
- IV NaHCO₃ to alkalinize urine (urine pH > 7.5) if alkalosis not present
- Anticipate hypokalemia and treat it (Diuresis → ↑Na⁺ load in CD → principal cells exchange K⁺ for Na⁺)
- If alkalosis or volume overload present, acetazolamide may be used for alkaline diuresis
- May cause acidosis if lost HCO₃⁻ not replaced
- Glucose containing fluid to prevent hypoglycemia (due to uncoupling of oxidative phosphorylation)
- Hemodialysis against a HCO₃⁻ dialysate in case of renal failure
- Ethylene glycol / methanol
- Saline or osmotic diuresis
- Thiamine & pyridoxine supplement
- Fomepizole (15 mg/kg loading dose) or IV ethanol (to achieve 100 mg/dl)
- Hemodialysis if pH < 7.3 or osmolal gap > 20 mosm/kg
- Renal failure
- Oral alkali replacement (1 - 1.5 mmol/kg)
- Shohl's solution (Na-citrate, causes aluminum toxicity if given with aluminium containing antacids)
- NaHCO₃ tablets
- Furosemide (60 - 80 mg/day) if hyperkalemia present
- Oral alkali replacement (1 - 1.5 mmol/kg)
NAGMA
Characterized by ↓HCO₃⁻ and normal AG. The loss of HCO₃⁻ is matched by reciprocal change in Cl⁻.
Etiology
- GI loss of HCO₃⁻
- Diarrhoea
- External pancreatic or small bowel drainage
- Ureterosigmoidostomy, jejunal loop, ileal loop etc.
- Drugs
- Calcium chloride (acidifying agent)
- Magnesium sulfate (diarrhoea)
- Cholestyramine (bile acid diarrhoea)
- Renal acidosis
- Proximal RTA
- PCT fails to reabsorb HCO₃⁻
- Type 2 RTA
- Diseases: Wilson's disease, multiple myeloma, Sjögren syndrome
- Drug induced: acetazolamide, topiramate
- Hypokalemia present
- Distal RTA
- α-inercalated cells of CD fails to secrete H⁺
- Type 1 RTA (Fanconi syndrome)
- Diseases: Sjögren syndrome, lupus, RA
- Drug induced: amphotericin B, ifosfamide
- Hyperkalemia present
- Hyporeninemic hypoaldosteronism
- Aldosterone deficiency / resistance → ↑K⁺ → ↓availability of NH₃ to buffer H⁺ → ↓H⁺ excretion
- Type 4 RTA
- Drug induced: NSAID, β-blocker, cyclosporine
- Hyperkalemia present
- Proximal RTA
- Drugs in renal insufficiency (hyperkalemia)
- K⁺ sparing diuretics (amiloride, triamterene)
- Trimethoprim
- pentamidine
- ACE-I and ARB
- NSAID
- Cyclosporine & tacrolimus
- Other
- Acid loads (NH₄Cl, hyperalimentation)
- Loss of potential HCO₃⁻ (ketosis with ketone body excretion)
- Expansion acidosis (rapid saline administration)
- Hippurate
- Cation exchange resins
Approach
- History of any predisposing condition, drugs etc.
-
Calculate urinary anion gap (UAG)
`"UAG" = [Na⁺]_u + [K⁺]_u - [Cl⁻]_u`
UAG indicates urinary NH₄⁺ level. UAG is negative when NH₄⁺ is appropriately increased in acidosis.
- Exclude GI loss (↑NH₄⁺ in urine, -ve UAG)
- Differentiate types of RTA (↓NH₄⁺ in urine, +ve UAG)
RTA Type 1 Type 2 Type 4 Location CD PCT Adrenal Defect H⁺ secretion HCO₃⁻ reabsorption Aldosterone deficiency / resistance Acidemia Severe Moderate Mild C/F Short stature, rickets, hypercalcuria Asymptomatic Asymptomatic Serum K⁺ ↓ ↓ ↑ Urinary pH Always > 5.5 Variable Always < 5.5
Treatment
- HCO₃⁻ therapy
- `"HCO₃⁻ deficit" = 0.5 × "lean weight" × (24 - [HCO₃⁻])`
- Treat underlying cause
- Management of RTA
- Type-1 RTA
- HCO₃⁻ replacement = 1-2 meq/kg/day
- K⁺ supplement
- Type-2 RTA
- HCO₃⁻ replacement = 10-15 meq/kg/day
- K⁺ supplement to minimize hypokalemia of alkali therapy
- Type-4 RTA (correct hyperkalemia)
- Dietary K⁺ restriction (40-60meq/day)
- Loop diuretic ± NaHCO₃ (0.5-1 meq/kg/day)
- Fludrocortisone (50-500 mcg PO daily) if primary adrenal insufficiency
- Type-1 RTA
Metabolic Alkalosis
It is characterized by ↑HCO₃⁻. It has two phases —
- Generative stage: the initial defect
- Loss of H⁺
- Gain of HCO₃⁻
- Contraction of volume around fixed content of HCO₃⁻
- Maintenance stage: impairment of renal HCO₃⁻ excretion
- ↓GFR
- ↑HCO₃⁻ reabsorption due to ↓Cl⁻, ↓K⁺ and ↓volume
Etiology
- Loss of H⁺
- GI loss
- Vomiting
- Gastric aspiration
- Congenital chloridorrhoea
- Villous adenoma
- Renal loss
ECFV contraction ECFV expansion - K⁺ deficiency
- Normotension
- Secondary hyperreninemic hyperaldosteronism
- K⁺ deficiency
- Hypertension
- Mineralocorticoid excess
- Diuretics
- Posthypercapnic state
- Hypercalcemia / hypoparathyroidism
- Recovery from lactic acidosis / ketoacidosis
- Non-reabsorbable anions e.g. penicillin, carbenicillin etc.
- ↓ Mg⁺
- ↓ K⁺
- Bartter's syndrome (loss of function mutation in TALH)
- Gitelman's syndrome (loss of function mutation in Na⁺-Cl⁻ cotransporter in DCT)
High renin Low renin - Renal artery stenosis
- Accelerated hypertension
- Renin secreting tumor
- Estrogen therapy
- Primary hyperaldosteronism e.g. adenoma, hyperplasia, carcinoma
- Adrenal enzyme defects e.g. ↓11β-hydroxylase, ↓17α-hydroxylase
- Cushing's syndrome / Cushing's disease
- Licorice, carbenoxolone, chewing tobacco
- (ECFV expansion + hypertension + ↓K⁺) + hyporeninemic hypoaldosteronism
- Liddle's syndrome (gain of function mutation of renal Na⁺ channel)
- GI loss
- Gain of HCO₃⁻
- Acute alkali administration
- Milk-alkali syndrome
Approach
- History to find out any predisposing factor
- Assess —
- ECFV
- Recumbent and upright BP
- Serum K⁺, Mg⁺²
- Urine Cl⁻, Ca⁺²
- Renin-aldosterone system
- Chloride responsiveness
Chloride-responsive Chloride-unresponsive Urinary chloride < 20 meq/l > 20 meq/l System involved GI / hypovolemia Renal Saline infusion Good response No response - D/D of renal causes
Chronic hypertension + chronic ↓K⁺ - Hypertensive patient on diuretics
- Mineralocorticoid excess
- Low plasma renin
- Normal urinary Na⁺ & Cl⁻
Normotensive + ↓K⁺ - Bartter's syndrome
- Gitelman's syndrome
- ↓Mg⁺
- Vomiting
- Exogenous alkali
- Diuretics
Alkaline urine + ↑Na⁺ + ↑K⁺ + ↓Cl⁻ - Vomiting
- Alkali ingestion
Acidic urine + ↓Na⁺ + ↓K⁺ + ↓Cl⁻ - Prior vomiting
- Posthypercapnic state
- Prior diuretic ingestion
Acidic urine + normal Na⁺, K⁺, Cl⁻ - ↓Mg⁺
- Bartter's syndrome
- Gitelman's syndrome
- Current diuretic ingestion
Differentiating features of Bartter's, Gitelman's and Liddle's syndrome —
Bartter's syndrome Gitelman's syndrome Liddle's syndrome Location TALH DCT CD Defect Loss of function mutation of Na-K-2Cl symporter Loss of function mutation of Na-Cl symporter Gain of function mutation of ENaC Mimics Loop diuretic Thiazide Hyperaldosteronism BP Normal/↓ Normal/↓ ↑ Aldosterone ↑ ↑ Normal/↓ Serum Na⁺ ↓ ↓ ↑ Serum K⁺ ↓ ↓ ↓ Serum Mg⁺² Normal ↓ Normal Urine Ca⁺² Normal ↓ Normal
Treatment
- Correct underlying pathology e.g. primary hyperaldosteronism, renal artery stenosis, Cushing's syndrome etc.
- Reduce GI / renal H⁺ loss with PPI, stop diuretics.
- Correct hypokalemia.
- If Cl⁻ responsive, resuscitate with saline infusion until euvolemia achieved.
- If saline infusion contraindicated, acetazolamide to promote renal HCO₃⁻ loss.
- Dose: 250 mg every 6h × 4 or 500 mg single dose.
- Isotonic HCl (150 meq/L) can be infused if alkalosis is severe (pH > 7.70).
- `"Required HCl" = 0.5 × "lean body weight" × ([HCO₃⁻] - 24)`
- Through central line over 8-24 hours.
- May cause hemolysis.
- If renal function impaired, hemodialysis against a ↓HCO₃⁻ & ↑Cl⁻ dialysate.
Respiratory Acidosis
It is characterized by ↑PaCO₂ due to hypoventilation.
Etiology
- Central
- Drugs (anesthetics, morphine, sedatives)
- Stroke
- Infection
- Airway
- Obstruction
- Asthma
- Parenchyma
- Emphysema
- Pneumoconiosis
- Bronchitis
- ARDS
- Barotrauma
- Neuromuscular
- Poliomyelitis
- Kyphoscoliosis
- Myasthenia
- Muscular dystrophy
- Miscellaneous
- Obesity
- Hypoventilation
- Permissive hypercapnia
Approach
- Detailed history and physical examination
- Signs / symptoms
- Initially headache, restlessness
- Progress to generalized hyperreflexia / asterixis, coma
- In chronic - sleep disturbances, memory impairment, motor disturbance
- Acute vs chronic
- Can be determined from amount of metabolic compensation
- Exclude pulmonary cause (PFT)
- Spirometry
- DLCO
- Lung volumes
- SaO₂ & PaCO₂
- Identify any non-pulmonary cause
- Detailed drug history
- Hematocrit
- Assess upper airway, chest wall, pleura, neuromuscular function
Treatment
- Acute
- Restore adequate alveolar ventilation
- Tracheal intubation
- Assisted mechanical ventilation
- Treat the underlying cause
- Restore adequate alveolar ventilation
- Chronic
- Carefully titre oxygen administration
- Injudicious use of oxygen may cause respiratory acidosis
- Gradually lower PaCO₂
- Rapid lowering of PaCO₂ may cause cardiac arrhythmia, ↓cerebral perfusion, seizures
- Provide sufficient Cl⁻ & K⁺ to enhance renal HCO₃⁻ excretion in order to prevent alkalosis
- Treat underlying cause and improve lung function
- Avoid HCO₃⁻ in simple respiratory acidosis
- HCO₃⁻ combines with H⁺ to form CO₂ which cannot be blown off due to ↓ventilation
- Carefully titre oxygen administration
Respiratory Alkalosis
It is characterized by ↓PaCO₂ due to hyperventilation.
Etiology
- CNS stimulation
- Pain
- Anxiety / psychosis
- Fever
- CVA
- Meningitis /encephalitis
- Tumor / Trauma
- Tissue hypoxia
- High altitude
- Pneumonia / pulmonary edema
- Aspiration
- Severe anemia
- Drugs / hormones
- Pregnancy / progesterone
- Salicylates
- Cardiac failure
- Chest receptor stimulation
- Hemothorax
- Flail chest
- Cardiac failure
- Pulmonary embolism
- Miscellaneous
- Septicemia
- Hepatic failure
- Mechanical hyperventilation
- Heat exposure
- Recovery from metabolic acidosis
Approach
- Acute vs Chronic
- Acute and chronic respiratory alkalosis can be differentiated by evaluating the adequacy of metabolic compensation.
- Acute respiratory alkalosis
- ↓Cerebral blood flow → light headedness, impaired consciousness
- ↑Generalized membrane excitability → seizures, arrhythmias
- ↓Ionized calcium → acute hypocalcemia
- ↓Na⁺, ↓K⁺, ↓PO₄⁻² due to intracellular shift
- ↑Cl⁻
- Left shift of Hb-O₂ dissociation curve (Bohr effect) → Cardiac arrhythmia in patients with heart disease
- Chronic respiratory alkalosis
- Common in critically ill patients
- Normocapnia + hypoxemia + hyperventilation ⇒ may indicate onset of respiratory failure
Treatment
- Correct underlying disease
- Adjust ventilator settings to minimize hypocapnia
- In hyperventilation syndrome - reassurance, rebreathing from paper bag
- Avoid antidepressants, sedatives
- β-adrenergic blockers may help reduce peripheral manifestations
Links
- Henderson-Hasselbalch equation
- Renal regulation of acid-base balance
- Collecting arterial blood sample for ABG analysis
- Management of diabetic ketoacidosis
References
- Harrison's Principles of Internal Medicine, 19th Edition, Chapter 66, Pages 315-324
- The Washington Manual of Medical Therapeutics, 34th Edition, Chapter 12, Pages 433-441
 RSS Feed
RSS Feed